A CADASTER report “Application of QSAR models for probabilistic risk assessment” has recently been accomplished. This report is a deliverable within the CADASTER project with an aim to show how to increase the use of non-testing information for regulatory decision whilst meeting the main challenge of quantifying and reducing uncertainty. The report contains examples of the integration of testing and non-testing information into assessment models for carrying out safety-, hazard- and risk assessments. The application of QSAR models for probabilistic risk assessment was discussed in respect to the characterization of uncertainty in QSAR predictions, the propagation of uncertainty in the assessment and sensitivity analysis of individual QSAR predictions with regard to their contributions in the overall risk assessment framework. Integration of QSARs in chemical safety assessment must acknowledge that treatment of uncertainty is context dependent, and uncertainty is to be interpreted in relation to the kind and quality of background information.
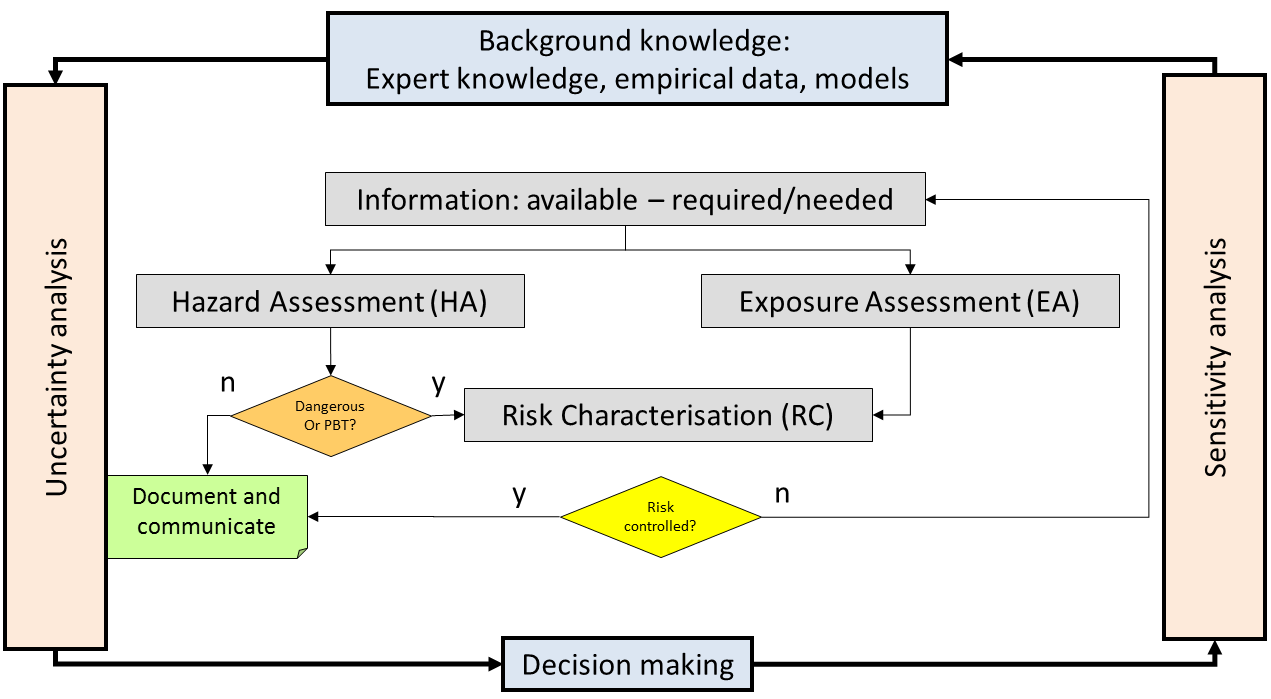
Two case-studies were used to demonstrate the computational framework for QSAR based risk assessment. The lesson learnt was that the application of QSARs in probabilistic risk assessment leads to questions such as:
- Are there any QSAR data available to use as weight-of-evidence of a chemico-specific input parameter to the exposure and effect assessments?;
- Which validated algorithm for prediction and approach for predictive inference will be used to support the uncertainty analysis?
- Is a QSAR prediction reliable enough to support the intended decision making?
The first case-study was an uncertainty and sensitivity analysis of QSAR and QSPR based-risk assessment on five Triazoles. Soil sorption partition coefficients, solubility, melting point, vapor pressure and hydroxyl radical reaction in air were predicted with identified QSPRs, and no effect concentrations with available QSARs developed for Triazoles. Parameter uncertainty was treated as a probability distribution for the chosen QSAR algorithm, for example a t-distribution when the QSAR algorithm was an ordinary least square regression. Exposure and effect assessment were carried out in the multimedia fate model Simplebox, and uncertainty was propagated by Monte Carlo simulation. Uncertainty analysis showed that the maximum permissible emissions to agricultural soil were highest for the Triazoles Bromuconazole and Difenoconazole. Sensitivity analysis, aimed to find sources of uncertainty with a large influence on the uncertainty in assessment output, found that uncertainty in maximum permissible emissions to agricultural soil was mainly determined by uncertainty in the soil sorption partition coefficient, the biodegradation in water and the toxicity.
The second case-study compared the discrepancy between non-testing versus testing-based risk assessments on three polybrominated diphenyl ethers. Exposure (PEC) and effect (PNEC) were assessed based on QSPR and QSAR predictions, treated in the same way is the first case-study except that variability in effect was considered through a species sensitivity distribution. Whenever available, parameters for the exposure assessment and species effect concentrations were replaced by information from experimentally tests on the chemicals in question. Discrepancies between non-testing and testing-based risk assessments were evaluated by studying the range of emissions for which the resulting decisions were different. We found that non-testing versus testing based risk assessments were different, but this difference depend to a large extent to how uncertainty is dealt with. The difference is small when predictions are precise, i.e. providing a good but balanced coverage of the experimental values, non-testing information are useful complement to reduce uncertainty in existing testing information of effects. However, there is uncertainty in experimental data which are not accounted for in QSAR modeling. The larges discrepancies could however be related to the use of uncertainty factors. For example, PNEC values were given a different treatment of uncertainty depending on the number of species with available information, where QSAR predictions increased the number of covered species. Also, experimental information was available for acute effects, whereas QSAR predictions were available for chronic effects, was another cause for difference in the treatment of uncertainty.
With respect to treatment uncertainty in QSAR predictions for probabilistic risk assessment the conclusions in the report are that:
- The integration of QSARs into probabilistic risk assessment is possible given proper assessments of predictive uncertainty and predictive reliability. Predictive uncertainty and reliability are identified to inform the characterization of parameter and model uncertainty, two kinds of uncertainty to be identified in probabilistic risk assessment. Uncertainty in probabilistic chemical safety assessment can roughly be divided in to three categories: parameter uncertainty, model uncertainty and scenario uncertainty. According to ECHA scenario uncertainty is “the uncertainty in specifying the scenario(s) which is consistent with the identified use(s) of the substance”. Scenario uncertainty is of less relevance for the uncertainty related to a QSAR. The other two terms, parameter and model uncertainty, are not frequently used in QSAR modeling. Instead a distinction lies between predictive uncertainty and predictive reliability.
- Probabilistic risk assessment is supported by QSAR predictions derived from Bayesian predictive inference. Predicting must be done with care, and the use of different bases for predictive inference is possible when a QSAR is treated as a scientific based hypothesis supported by empirical data. In other words, the suggestion is to treat QSAR data that has been validated for its predictive performance as the QSAR to base predictions on. It is up to the assessor to choose an appropriate method for predictive inference, given that performance measures of predictivity do not deviate to much from the measures in the peer-reviewed validation. This will result in a more flexible use of QSARs with a possibility of updating as long as new QSAR data becomes available.
- The extent of extrapolation in a QSAR prediction influences predictive error and predictive reliability, and the domain of applicability is from an applied perspective context dependent and considered in the treatment of uncertainty. A separation between predictive uncertainty and predictive reliability makes it possible to both apply models and discuss their reliability in a constructive way.
This report focuses on predictions of chemical specific properties and activities to replace testing information in chemical regulation. Statistical inference may have different purposes, and when QSARs are applied to support decisions making based on unobserved quantities such as in risk assessment, drug development, or experimental design, the statistical problem is to make predictions, referred to as predictive inference. Predictive science involves the use of a belief system about observables in science, and a philosophy of scientific methodology that implements that belief system. Predicting should be done with care and there are (solvable) practical problems when the purpose of statistical inference changes from inference on models to inference on predictions.
The report discusses three kinds of philosophies for predictive inference of relevance for the application of QSARs in probabilistic risk assessment. Sampling Theory estimate predictive uncertainty based on a representative sample. Such (frequentist) inference rests upon assumptions of independent and, for example, identically distributed observations, in combination with a probabilistic assumption of uncertainty. Under violence of any of these assumptions, appliers of frequentist inference run into problems.
The Bayesian paradigm for inference
assign, instead of
assume, a probabilistic model for observations, and assign models for uncertainty in parameters (so called priors). Bayesian inference uses Bayes rule to update expert knowledge with information in empirical observations. The result is a well-defined probabilistic model of uncertainty. In cases of doubts, the caveat is the necessity to choose priors and probabilistic models (likelihoods). For example, there is no need to check an assumption of normality of errors (as in the frequentist case), as this is assigned through expert judgment.
The third alternative is to assign a probability distribution for predictive uncertainty based on expert judgment only. This can for example, be based on experience of experimental testing, or based on combinations of different sources of information.
Sampling Theory and solid expert judgment can be seen as extremes kinds of Bayesian inference; the first as Bayesian inference with non-informative priors (expressed simplistically, but it is more difficult than that); the second as Bayesian inferences with only priors. Therefore the recommendation we give is to use Bayesian inference as the statistical philosophy for predictive inference when QSARs are applied in probabilistic risk assessment.
 The second CADASTER workshop will be organized in
The second CADASTER workshop will be organized in  Recently, the HEROIC project has been initiated within the FP7 framework. “HEROIC” is the acronym of “Health and environmental risks: organisation, integration and cross-fertilisation of scientific knowledge”. The project is aimed at bridging the gap between human and environmental risk assessment and wishes to contribute to the harmonisation of tools and methods adopted in human and environmental risk assessment, thus reducing animal testing and resource use in general and facilitating better co-operation between risk assessors in both disciplines.
[
Recently, the HEROIC project has been initiated within the FP7 framework. “HEROIC” is the acronym of “Health and environmental risks: organisation, integration and cross-fertilisation of scientific knowledge”. The project is aimed at bridging the gap between human and environmental risk assessment and wishes to contribute to the harmonisation of tools and methods adopted in human and environmental risk assessment, thus reducing animal testing and resource use in general and facilitating better co-operation between risk assessors in both disciplines.
[ In the beginning of 2009 the work on the Cadaster project began with the collection of existing experimental data. Data search on all endpoints of relevance was performed for the environmental risk and hazard assessment of the groups of chemicals included in the case studies. The survey of the existing QSAR/QSPR models was performed for the four classes of chemicals selected in this project (PBDEs, PFAs, fragrances and (B)TAZs). An overview was prepared of the non-testing options given under REACH to either replace experimental testing, or to strengthen confidence in experimental results (QSARs, read-across, category approaches, and exposure based waiving). An extended database on existing experimental data and (Q)SAR models was created for the four selected group of chemicals.
[
In the beginning of 2009 the work on the Cadaster project began with the collection of existing experimental data. Data search on all endpoints of relevance was performed for the environmental risk and hazard assessment of the groups of chemicals included in the case studies. The survey of the existing QSAR/QSPR models was performed for the four classes of chemicals selected in this project (PBDEs, PFAs, fragrances and (B)TAZs). An overview was prepared of the non-testing options given under REACH to either replace experimental testing, or to strengthen confidence in experimental results (QSARs, read-across, category approaches, and exposure based waiving). An extended database on existing experimental data and (Q)SAR models was created for the four selected group of chemicals.
[ In WP3 several local models for the selected classes of CADASTER chemicals have been developed, presented to various international meetings and
In WP3 several local models for the selected classes of CADASTER chemicals have been developed, presented to various international meetings and 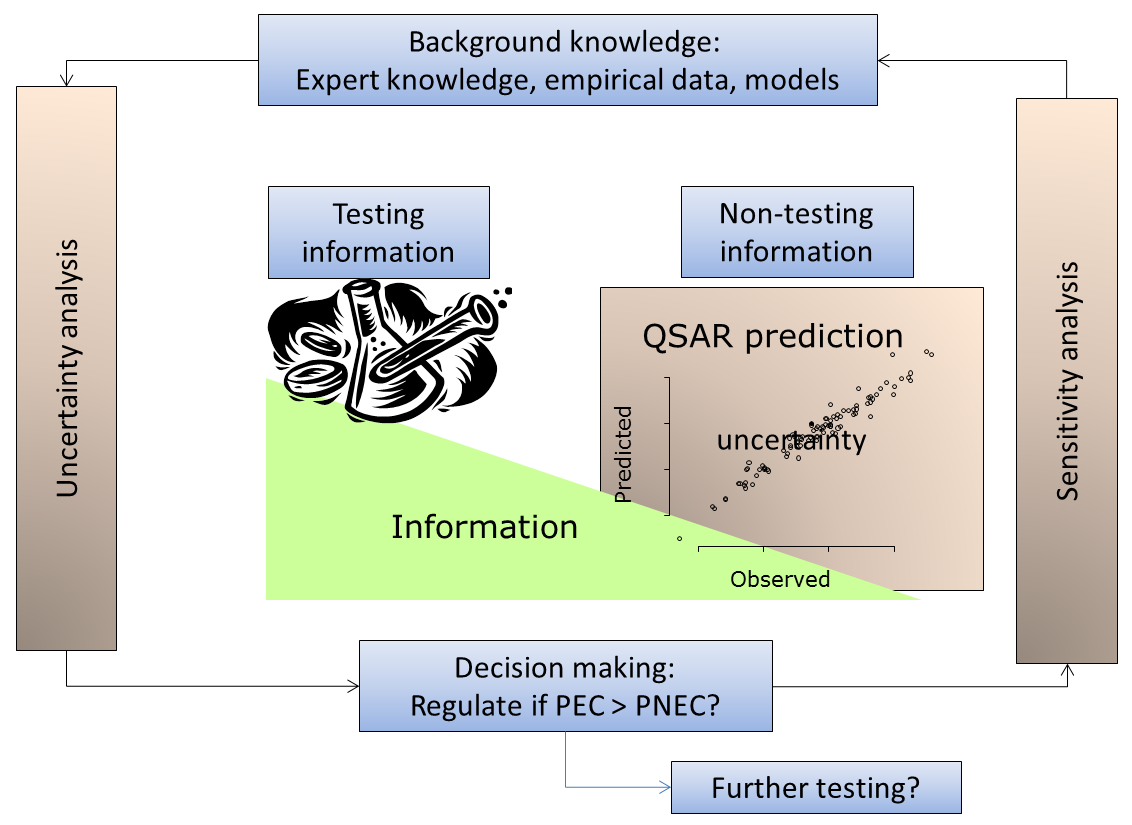 Chemical legislation allows the use of QSARs to support or replace experimental testing risk assessment. Risk assessment asks for a quantification of uncertainty, which is to be understood and interpreted in relation to the background knowledge. There are solved and unsolved issues on how to treat uncertainty when going from testing to non-testing information in probabilistic risk assessment.
[
Chemical legislation allows the use of QSARs to support or replace experimental testing risk assessment. Risk assessment asks for a quantification of uncertainty, which is to be understood and interpreted in relation to the background knowledge. There are solved and unsolved issues on how to treat uncertainty when going from testing to non-testing information in probabilistic risk assessment.
[ Two case-studies were used to demonstrate the computational framework for QSAR based risk assessment. The lesson learnt was that the application of QSARs in probabilistic risk assessment leads to questions such as:
Two case-studies were used to demonstrate the computational framework for QSAR based risk assessment. The lesson learnt was that the application of QSARs in probabilistic risk assessment leads to questions such as:
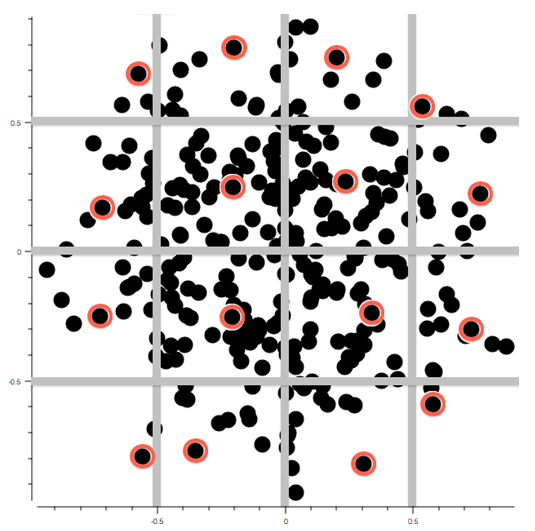 A crucial step in modelling and risk assessment is the reliable selection of a representative subset of chemical compounds. Within its contribution to WP3 and WP4 and in collaboration with LNU, the HMGU recently published two studies comparing several commonly used approaches for experimental design in QSAR modeling with newly developed ones evaluating their efficiency and reliability. The first study introduces an adaptive, stepwise approach, that combines the D-Optimal selection criterion for linear models with partial least squares techniques, the second study focuses on the usage of the k-Medoid clustering for experimental design in chemoinformatics. Both approaches have been shown to significantly improve the performance of resulting models. This is of basic interest both in terms of efficiency and to save time and costs.
[
A crucial step in modelling and risk assessment is the reliable selection of a representative subset of chemical compounds. Within its contribution to WP3 and WP4 and in collaboration with LNU, the HMGU recently published two studies comparing several commonly used approaches for experimental design in QSAR modeling with newly developed ones evaluating their efficiency and reliability. The first study introduces an adaptive, stepwise approach, that combines the D-Optimal selection criterion for linear models with partial least squares techniques, the second study focuses on the usage of the k-Medoid clustering for experimental design in chemoinformatics. Both approaches have been shown to significantly improve the performance of resulting models. This is of basic interest both in terms of efficiency and to save time and costs.
[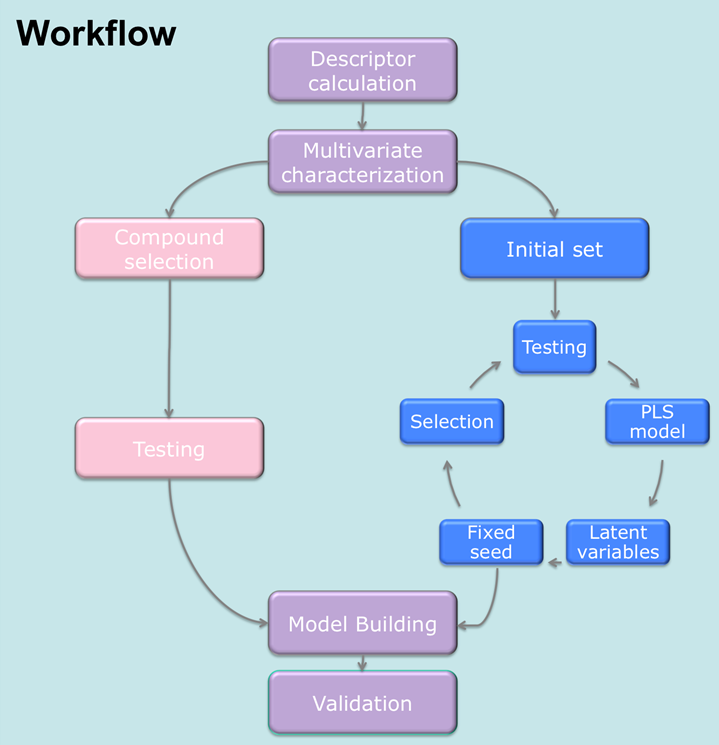 In our first study, we showed that a stepwise refinement of the representation of the chemical space can be used to significantly decrease the number of required experiments. This refinement is implemented by taking the growing amount of information, which is gathering within the experimental testing cycles, into consideration. The correlation between the measured property and the descriptor space is thereby the criterion for the rearrangement of the search space. To evaluate the newly developed idea, we compared the performance of models derived with the D-Optimal criterion such a representation of the chemical space to that of models derived using the same selection criterion, but applied in a static manner on principal components. The validation was done on four datasets with different endpoints, representing toxicity, physicochemical properties and an adsorption coefficient. Compared to models derived from the selection on principal components, the models derived from the selection on PLS latent variables had a lower RMSE and a higher Q2 and an increased correlation coefficient. Further, we were able to produce models of the same quality with testing only 60% of compounds.
In our first study, we showed that a stepwise refinement of the representation of the chemical space can be used to significantly decrease the number of required experiments. This refinement is implemented by taking the growing amount of information, which is gathering within the experimental testing cycles, into consideration. The correlation between the measured property and the descriptor space is thereby the criterion for the rearrangement of the search space. To evaluate the newly developed idea, we compared the performance of models derived with the D-Optimal criterion such a representation of the chemical space to that of models derived using the same selection criterion, but applied in a static manner on principal components. The validation was done on four datasets with different endpoints, representing toxicity, physicochemical properties and an adsorption coefficient. Compared to models derived from the selection on principal components, the models derived from the selection on PLS latent variables had a lower RMSE and a higher Q2 and an increased correlation coefficient. Further, we were able to produce models of the same quality with testing only 60% of compounds.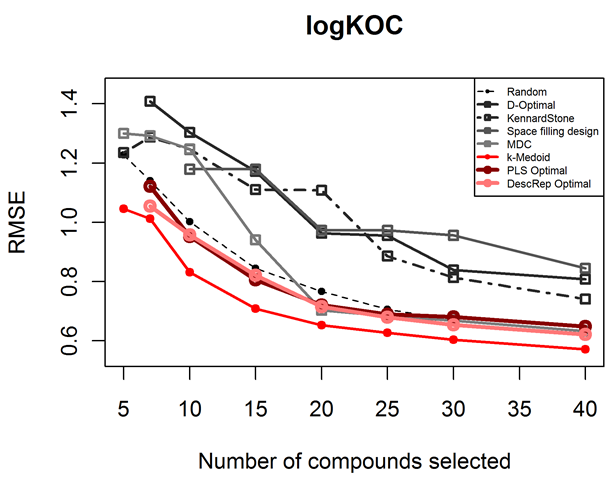 As a stepwise selection process is not feasible for all problems, we additionally investigated on the most efficient non-stepwise selection criterion. We compared approaches like the classical D-Optimal criterion, the Kennard-Stone algorithm, full factorial design, a representativity selection to a selection based on the k-Medoid clustering. The results show that the performance of models developed on selections based on the k-Medoid approach is among the best for all examined datasets. It is the only approach to deliver significantly better results on all datasets than a random selection. Compared to the models derived from the compounds selected by the other examined approaches, those derived with the k-Medoid selection show a high reliability for experimental design, as their performance was constantly among the best for all examined datasets. The reasons for this exceptional performance are:
As a stepwise selection process is not feasible for all problems, we additionally investigated on the most efficient non-stepwise selection criterion. We compared approaches like the classical D-Optimal criterion, the Kennard-Stone algorithm, full factorial design, a representativity selection to a selection based on the k-Medoid clustering. The results show that the performance of models developed on selections based on the k-Medoid approach is among the best for all examined datasets. It is the only approach to deliver significantly better results on all datasets than a random selection. Compared to the models derived from the compounds selected by the other examined approaches, those derived with the k-Medoid selection show a high reliability for experimental design, as their performance was constantly among the best for all examined datasets. The reasons for this exceptional performance are:
 The
The  The
The